Medical devices range from everyday products such as adhesive plasters, fever thermometers, and blood pressure monitors to hip implants and complex devices such as X-ray and magnetic resonance tomography devices. In order to be placed on the market in the EU, these products must comply with European regulations and be labelled with the CE mark. The legal basis for this is Regulation (EU) 2017/745 on medical devices (MDR – Medical Device Regulation).
A comprehensive overview of the steps required for CE marking can be found in our brochure “10 Steps to CE Mark.”
On this page you will find more information about the certification services offered by SGS through its Notified Bodies SGS Fimko (CE 0598) and SGS Belgium (CE 1639).

Conformity assessment by a Notified Body
If a medical device belongs to a higher risk class than Class I, its conformity with the requirements of the MDR must be assessed by a Notified Body.
Depending on the risk class, there are different pathways for conformity assessment. Our infographic illustrates the possible procedures in accordance with MDR Article 52 and highlights key aspects to consider when preparing for the conformity assessment.
We recommend the route via Annex IX (conformity assessment based on a quality management system (QMS) and assessment of the technical documentation), as the MDR requires manufacturers of medical devices to implement a QMS that meets minimum requirements.
Certification process with SGS
If you are planning to have your medical device certified with the support of a Notified Body (corresponding to steps 6 and 7 in the above-mentioned brochure “10 Steps to CE Mark”), feel free to contact us. You will receive the necessary application documents as well as further information on the certification process, which consists of several steps (A to G), starting with your application.
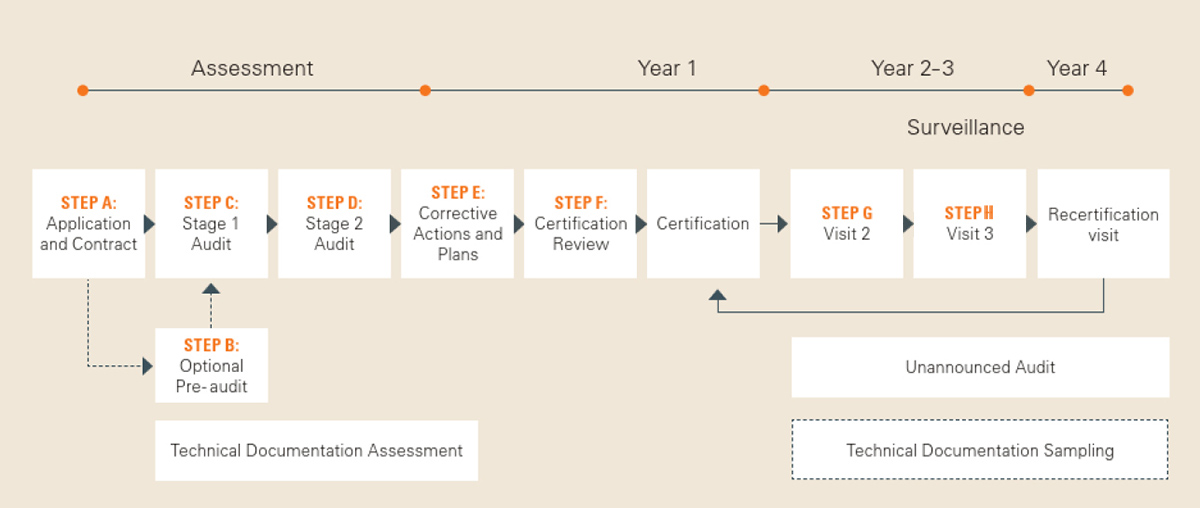
Our brochure “Your Certification Process Explained” provides a detailed overview of these steps in advance.
Since medical devices and manufacturers vary greatly, it’s not possible to provide a reliable estimate of timelines or costs without reviewing your application. However, our MDR standard fees list offers an initial overview of the expected costs.
Additional information
The MDR (EU) 2017/745 as a legal framework can be challenging to interpret. For this reason, the Medical Devices Coordination Group (MDCG) publishes a series of MDCG Guidance Documents.
These documents provide practical recommendations on various topics – from EUDAMED and classification issues to the interpretation of significant changes. Although the MDCG documents are not legally binding, they are applied by Notified Bodies and are highly recommended as supplementary reading.
Downloads
- 10 Steps to CE Mark
Fact sheet outlining the 10 essential steps required to obtain the CE mark for a medical device (CE certification) - Conformity Assessment Routes
Infographic illustrating the different available conformity assessment routes under MRD - Your Certification Process Explained
Medical Device Certification – this document explains the steps of the initial certification process and the audit cycle in accordance with the MDR - MDR Client technical documentation submission checklist
Index and structure of the technical documentation to be submitted - MDR Standard Fees List
Costs for the certification process
Contact
Armin Hudetz
t: +49 89 78 74 75-133
E-Mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Customer Service Team
t: +49 89 78 74 75-222
E-Mail: This email address is being protected from spambots. You need JavaScript enabled to view it.




 SGS has several certification bodies according to the European Directives.
SGS has several certification bodies according to the European Directives. Notified Body MED
Notified Body MED